 |
 |
| Home | Welcome | What's New | Site Map | Glossary | Weather Doctor Amazon Store | Book Store | Accolades | Email Us |
 | |||||||||
The Joy of SnowflakesThough many will try to tell you that the Winter Solstice, the astronomical event when the noonday sun has stands overhead at the Southern Hemisphere's Tropic of Cancer, "officially" begins winter, there is no set start date for weather (though most climatologists will take December 1 as a convenient start) For me, I think of winter as being ushered in with the first snowfall that sticks to the ground for several days. To reach that day, we must accumulate billions of snowflakes on the local terrain — a unique assortment of crystalline water.
Snowflakes typically form when near-surface air temperatures are not far from the freezing mark. At these temperatures, snow crystals are more "sticky," and those that collide together will adhere to one another better than at colder temperatures. At very cold surface air temperatures, they do not stick together well at all, and thus bitter-temperature snowfalls are mostly comprised of snow crystals. Snow CrystalsSnow crystals are formed of ice molecules and are typically 0.5 to 5 millimetres (0.02 to 0.20 inches) in size. Snowflakes are typically bigger — generally about 10 mm (0.4 inches) across and perhaps as large as 20 to 40 mm (0.79 to 1.57 inches). Exceptionally large snowflakes can exceed 50 mm (2 inches) and aggregate hundreds of individual crystals. For a snowflake to grow exceptionally large, however, conditions must be perfect. Besides ideal temperature needed for stickiness, large flakes usually only grow when winds are light; otherwise the large flakes will break up as they fall. The biggest snowflake reportedly measured 15 inches (38 cm) across and fell on January 28, 1887 at Ft Keough, Montana. Another giant fell in Bratsk, Siberia in 1971, a flake 20 cm (8 inches) by 30 cm (12 inches). Two decades earlier, residents of the English town of Berkhamsted saw snowflakes big as saucers, almost 13 cm (5 inches) across. We think snowflakes, or more correctly snow crystals, are six-sided, frilly stars and are fairly flat like a pizza. Snow-crystal structure, however, takes on many forms. Besides the six-armed stars that look like frozen lace, there are a variety of shapes from flat hexagonal plates to three-dimensional small hexagonal pillars with hexagonal plates lying on each end, popularly called cufflink crystals. Other snow crystals resemble Dorian columns; some look like oak leaves; some are shaped like dinner plates.  Snow Crystal ClassesNormally, however, such perfectly formed crystals do not survive the fall to earth. Most crystals break off arms as they are buffeted by winds within a cloud before they grow to sufficient size to fall, producing irregular and unsymmetrical shapes. Snow Crystal FormationScientists puzzled over why snowflakes have six sides for centuries. In 1611, famed astronomer Johannes Kepler took time away from peering at the heavens to look into the small snowflakes of Earth. Writing in an essay "On the six-cornered snowflake," Kepler postulated that snowflakes were made up of globules of condensed and frozen moisture — their symmetry arising from geometrical efficiency because they were well-packed arrays of tiny spheres. 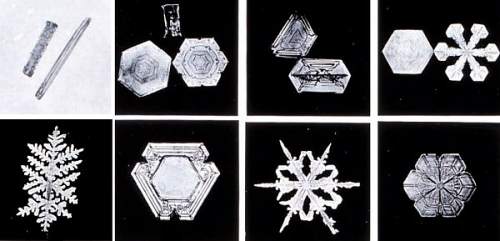 It took more than three centuries to fully correct Kepler's concept. We now understand that the shape of a snowflake is first determined at the molecular level by the atoms composing the water molecule. Each molecule contains two atoms of hydrogen joined to one atom of oxygen — H2O. In water, oxygen has a more powerful hold on the electrons that the two elements share in their water-making bond. As a result of oxygen's tighter grip, the oxygen atom side of the water molecule becomes slightly more negative while the hydrogen side becomes slightly more positive. Since opposite charges attract, the positive hydrogen atom of one water molecule tends to stick to the oxygen atom of a neighbouring molecule. In liquid and vapour water phases, water molecules jostle and dance around their neighbours, forming few stable links between molecules. But when a mass of water molecules loses enough heat energy — that is, as the water cools down toward the freezing point — the bonds (called hydrogen bonds) between water molecules form more readily and break less often. 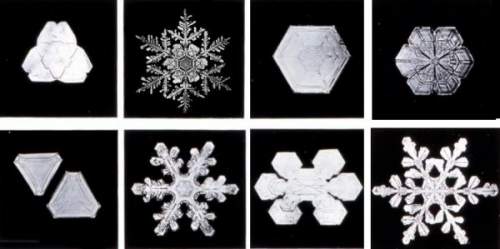 Upon freezing, everything seizes up. Now, each water molecule is surrounded tetrahedrally by four others to which it bonds. The oxygen atoms are arranged hexagonally in layers. When a new ice forms on the growing crystal's sides, it spends less energy than if it were to add a layer on its top or bottom. The side faces advance more quickly, and the crystal structure forms. This is what provides the underlying sixfold symmetry of the crystal lattice, that grows to become the visible snow crystal or platelet of ice. Ice crystals are sensitive to the conditions under which they form, particularly the air temperature, and the excess relative humidity expressed as the supersaturation level — both within and without the clouds. These factors not only affect how the crystals grow but the basic shape they take. The colder the temperature, the sharper the ice crystal tips. At warmer temperatures, the ice crystals grow more slowly and smoothly resulting in less-intricate shapes, i.e., more needles and plates. Thus, dendrite crystals generally form in high clouds, needle or flat six-sided crystals often originate in middle height clouds, and a wide variety of shapes grow in low clouds.  For example, thin, hexagonal plate-like snow crystals form in air at 0 to -3.8o C (32o to 25 o F). In colder air (-3.8o to -6.1o C / 25o to 21o F), needle shapes occur. Long, hollow hexagonal columns appear from -6.1o to -10o C (21o to 14o F). Flower-like plates form at temperatures from -10o to -12.2o C (14o to 10o F). Six-pointed stars, called dendrites, appear from -12.2o to -16.1o C (10o to 3o F). Wilson Bentley, The Snowflake Man of Jericho, Vermont
Bentley became a local, and then national, legend and is still today known as "Snowflake" Bentley and "The Snowflake Man." His 1931 book Snow Crystals (from which the crystals show here are taken)contains more than 2400 snow crystal images from his vast collection. It is likely that the belief that no two snowflakes are alike stems from this passage from a 1925 report by Bentley: "Every crystal was a masterpiece of design and no one design was ever repeated. When a snowflake melted, that design was forever lost." More on Bentley, his life and work can be found at the Wilson A. Bentley website. [A full biography has been written by my friend Duncan Blanchard (see below), but here is a condensation of Bentley's life by Blanchard.] For me, December snow storms often appear to have a magical quality not seen in later months. The year's first good snowfall appears to drift from clouds pregnant with large, fluffy flakes that cover the surrounding fields and meadows like a goose-down duvet. When temperatures hang around the freezing point, ironically, you can almost feel a warmth while surrounded by the falling flakes as winter is ushered in, transforming the dull brown landscape into an ermine fairyland, delicate and transient. (Snow photographs by Wilson A. Bentley, public domain.)Learn More From These Relevant Books
|
|||||||||
 |
To Purchase Notecard, |
Order Today from Amazon! | |
 |
 |
To Order in Canada: |
To Order in Canada: |


