 |
 |
| Home | Welcome | What's New | Site Map | Glossary | Weather Doctor Amazon Store | Book Store | Accolades | Email Us |
 | |||||||||||||||||||||||||||||
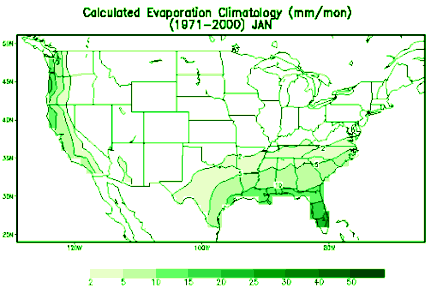
Weather Almanac for July 2010EVAPORATION: WATER’S DISAPPEARING ACTMidsummer, and things are drying out: forests, grasses, gardens. Although many areas across North America receive much of their annual precipitation during the summer months, the result of quick, heavy downpours during convective rainfall events, it is also the time for the greatest loss of water from the surface through the processes of evaporation and transpiration. Evaporation, in its most general expression, is the conversion of a liquid directly into a gas. In weather, it is the conversion of liquid water into water vapor. A close cousin to evaporation is evapotranspiration, which combines the evaporation from vegetation with the transpiration of water from photosynthesizing plant leaves. Transpiration takes into account water lost to the atmosphere by water movement within a plant from the soil to its subsequent loss as vapor through the stomata in its leaves. Since the Earth’s surface is mostly covered by water, over 70 percent, it is not surprising that a large portion of the annual global evaporation comes from the surface waters — approximately 90 percent. The remaining 10 percent originates from the land and plant cover. Eventually, the water enters the precipitation process and falls as rain or snow, a major component of the global water cycle.  If we were to averaged evaporation across the entire planet, the amount of water leaving the surface would be about 3 millimeters per day (about 1/8 of an inch per diem). The rate is much larger over the tropical oceans, and, conversely much lower over cold water surfaces. Over, deserts it is near zero, since there is little water, if any, available for evaporation. The PhysicsEvaporation and boiling are two processes by which a liquid vaporizes, which is a change of phase from liquid to gas. In changing phase from liquid to gas, energy must be entered into the system. This is known as the latent heat of vaporization and amounts to 2260 kJ/kg or 540 calories/g. Whereas boiling is a rapid change of phase, requiring the temperature of the liquid to reach the boiling point before vaporization can occur, evaporation from a water surface proceeds relatively slow at temperatures below the boiling point. For water molecules to evaporate from a water surface, they must be near the air-water surface. There, given the right direction of movement and sufficient kinetic energy, some are able to overcome the forces keeping them in the liquid and they break free. The kinetic energy of a water molecule is proportional to its temperature, so warmer waters will evaporate faster than cooler ones. When water molecules break free, they take their energy from the water body and it, in response, cools, a process known as evaporative cooling. This is the cooling we feel when water, or perspiration, evaporates from our skin. 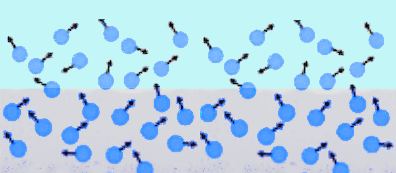 If we could look very closely at a water surface, we would see at the molecular level that it is not the definitive boundary we imagine but a fuzzy transition zone between the air above and liquid below. Watching this zone, we would see some molecules jumping out into the air, like rockets reaching escape velocity from the Earth, and others jumping out then falling back into the water body from the air (reentry, to continue our analogy). At equilibrium, as many molecules fall back as escape. But under the right conditions, more molecules escape than reenter, and we have water loss: net evaporation. Under other conditions, more molecules may fall back than escape and there is a net condensation, the opposite process to evaporation. Several environmental factors control the process and determine whether there is net evaporation or not. The three key parameters are heat energy, humidity and air movement (aka wind). Heat energy gives the molecules more kinetic energy and provides the impetus to break free. It also determines the temperature of the water body, and thus its vapor pressure. The higher the temperature, the higher the vapor pressure at the surface, and the higher the evaporation rate. Solar radiation provides much of the heat energy used in the evaporation process, thus sunnier areas and periods have the most evaporation. When the air above the water surface is dry, that is, it has low absolute humidity, fewer molecules are available to reenter the water, and thus there is a steady migration of water molecules from the surface into the air above and eventually away from the surface influence. With high humidity air, more water molecules in the air can return to the surface, and the evaporation rate is slow. As a result, evaporation rates are higher when dry air sits atop a water surface than when very humid air is near it. The third major factor is air movement or wind. Windier conditions near the surface tend to move water molecules away from it into the higher air and thus increase the evaporation rate over similar conditions with calm air. In effect, the wind physically removes molecules of evaporated moisture quickly away from the surface, allowing more evaporated water to refill the space. A 5 mph wind will increase evaporation by 20 percent over the still-air rate; a 15 mph wind by 50 percent. These factors drive evaporation from liquid waters surfaces like the oceans and lakes. In the contiguous United States, the Southwest receives the highest solar radiation and therefore has the greatest lake evaporation — the amount of water evaporating from a lake surface — while areas that receive the minimum solar radiation, the Northeast and Pacific Northwest, have the least lake evaporation. 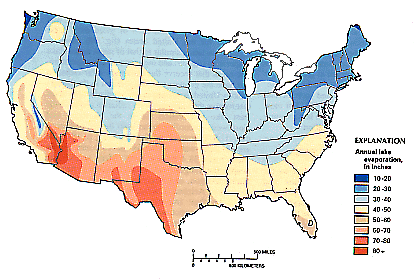 Annual lake evaporation in the conterminous United States, 1946-55. |
|||||||||||||||||||||||||||||
 |
 |
Evaporation station and check pan |
Observer measures the depth of water |
The observation day begins with the pan filled to exactly two inches (5 cm) from the pan top. Evaporation is then measured daily by determining the water depth at the end of the observation day and subtracting this value from the filled depth, the difference is the depth of water evaporates from the pan at the end of 24 hours. Then, the pan is refilled to the reference level. If precipitation has occurred in the preceding 24-hour period, its depth is accounted for in calculating the evaporation. Rainfall causes the most common and obvious errors, particularly if the daily rainfall event exceeds 55mm (2.2 inches), a situation when the Class A Evaporation pan will likely overflow.
The sunken Colorado pan, as its name implies, is sunk into the ground to within about 5 cm (2 in.) of its rim. It is square, 1 m (3 ft) on a side, and 0.5 m (18 in.) deep and made of unpainted galvanized iron. Like the sunken Colorado pan, the Symon’s pan is sunken in the ground and it has three times the surface area of the Class A pan.
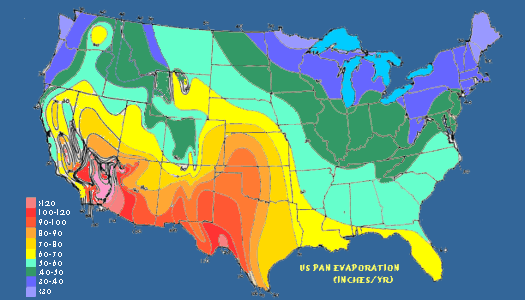
A important effect of vegetation on moisture transfer from the surface to the air manifests through the process of transpiration. It is the water loss through leaf stomata from plants. (Stomata are small openings on all above ground plant tissue, and are most abundant on the underside of leaves; they are the “nostrils” of plants —though the term derives from the Greek word for mouth.) Stomata allow the passage of carbon dioxide in and oxygen and water vapor out of the leaf. The process of transpiration is a plant’s equivalent, in some degree, to respiration in animals.
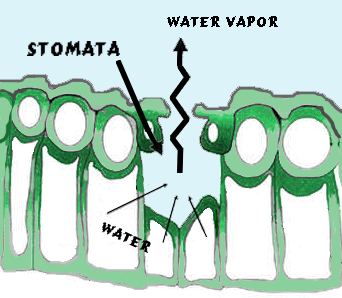
In the photosynthetic process, carbon dioxide is taken in through the stomata and oxygen is released. During this gaseous exchange, water vapor is also released at a rate dependent on the humidity of the air surrounding the leaf and the moisture content of the soil in which the plant roots reside. The process is largely passive, though some plants have the ability to close their stomata, but at the expense of its photosynthesis. As a result, plants face the dilemma of needing to intake carbon dioxide while losing needed water vapor through the stomata. If this water loss is too great, the plant can deplete its water reserve and thus shut down its photosynthetic factory.
Of the water passing through a plant only 1% is used in the growth process, the rest is transpired. For example, crops may transpire 200 to 1000 kg of water for every kg of dry plant matter produced. Transpiration also moves nutrients from the soil through the roots to the various plant cells and keeps plant tissues from overheating.
The amount of water lost to the atmosphere during transpiration depends to a large degree on the plant species, the amount of subsurface water available to the plant, and the atmospheric conditions. The quantity of water that plants can transpire varies with a number of factors that determine transpiration rates. The main factors are:
We can make a few generalizations on transpiration for various groups of plants. Herbaceous plants transpired less than woody plants because their root systems do not reach to great soil depth. Deciduous forests transpire less than coniferous ones due to their loss of leaves each year. However, when both are in full leaf, deciduous forests will generally transpire more than coniferous stands.
During the active growing season, a leaf transpires a quantity of water many times its own weight. For example, a typical acre of corn releases approximately 3,000-4,000 gallons (11,400-15,100 liters) of water each day to the air. A large oak tree may transpire 40,000 gallons (151,000 liters) per year.
Over a large vegetative surface, it is difficult to partition how much water enters the air through evaporation and how much through transpiration. Therefore, biometeorologists combine the two processes and call it evapotranspiration. The term is defined as the total water lost to the atmosphere from the ground surface by evaporation from the capillary fringe of the groundwater table, and the transpiration of groundwater by plants whose roots tap the groundwater table. Evapotranspiration involves the evaporation process from open pools of water, all wetlands, melting snow cover, and bare soil and the transpiration process from vegetation. Because of the importance of solar energy in the process, evapotranspiration varies with latitude, season of year, time of day, and cloud cover.
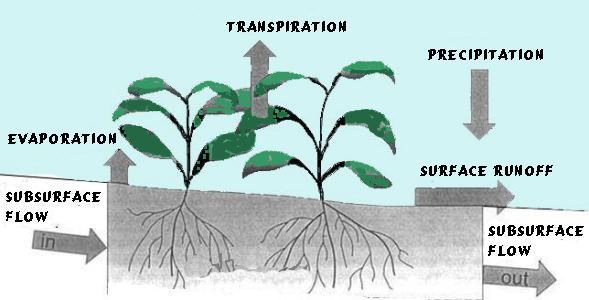
During a drought, the importance of evapotranspiration becomes magnified, because evapotranspiration depletes the limited remaining water supplies in lakes and streams and the soil.
A similar term important to biometeorologists and agronomists is potential evapotranspiration (PET) defined as the ability of the atmosphere to remove water that could be evaporated and transpired if there was unlimited water available. PET is expressed in terms of a depth of water in millimetres or inches. PET is usually measured indirectly using other measured climatic factors. When average annual PET is compared to average annual precipitation, P, their ratio P/PET is known as the aridity index. The table below gives the range of aridity index (AI) for several dry-land classifications.
| Classification | Aridity Index |
| Hyperarid | AI < 0.05 |
| Arid | 0.05 < AI < 0.20 |
| Semi-arid | 0.20 < AI < 0.50 |
| Dry subhumid | 0.50 < AI < 0.65 |
PET incorporates the available energy for evaporation and the lower atmosphere’s ability to transport evaporated moisture away from the land surface. It is determined as the evapotranspiration rate of a short green crop of uniform height, which completely shads the ground. Since only plant physiology can limit the transpiration rate; it is independent of any atmospheric or soil moisture restrictions. Therefore, PET is considered the maximum possible evapotranspiration rate for a given set of meteorological and physical parameters. Evapotranspiration can never exceed PET, but will be lower if there is not enough water to be evaporated or plants are unable to readily transpire.
In general, PET is higher in the summer and on windy days, and less on cloudy days. PET values are higher closer to the equator, because solar radiation is the main factor that drives evaporation. PET. Daily fluctuations in evapotranspiration rates also occur. On clear days, the rate of transpiration increases rapidly in the morning and reaches a maximum usually in early afternoon or mid-afternoon. The midday warmth can cause closure of plant stomata, which results in a decrease in transpiration
Hydrologists estimate mean annual evapotranspiration from the mean annual values of four principal components of the hydrologic budget: precipitation, surface-water inflow, surface-water outflow, and consumptive use. All four components were measured or estimated, and evapotranspiration computed as a residual of these components. As a example of the impacts of evapotranspiration on a region, we can look to the conterminous United States. Calculations indicate that evapotranspiration within the US ranges from about 10 inches per year in the semiarid Southwest to about 35 inches per year in the humid Southeast. In relation to precipitation, the estimated average statewide evapotranspiration are about 40 percent of the average annual precipitation in the Pacific Northwest and the Northeast, and about 100 percent in the desert Southwest.
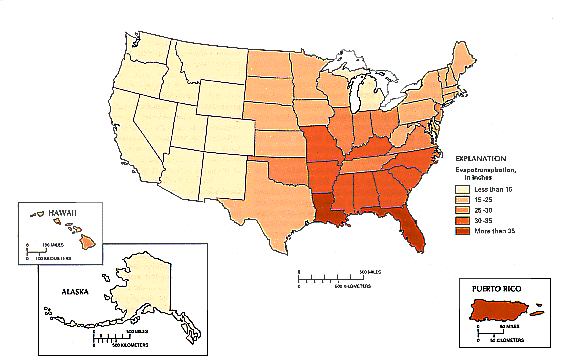
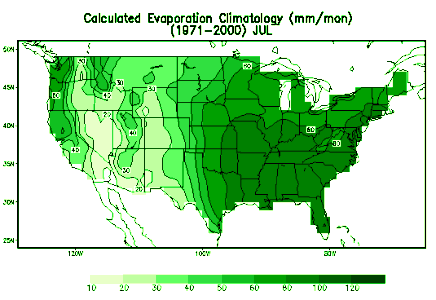
The United States’ seasonal variability in transpiration varies greatly across the nation in a manner similar to the seasonal air temperature trend. In the northern US, measurable transpiration, primarily from natural vegetation, usually begins in April, reaches a maximum in July, and then decreases in October. In contrast, the southern US sees transpiration continue throughout the winter months, though at a comparatively small rate, and typically it is greatest in the early summer months (June and July), when leaf area of plants is maximum.
 |
 |
Precipitation and evapotranspiration are the two major components in the hydrologic, or water, budget of most areas and for the planet. On the global scale, evapotranspiration equals precipitation over the year. When evapotranspiration exceeds precipitation over a long period, drought may ensue. Evapotranspiration changes during a drought depend largely on the moisture availability at its onset and the severity and duration of the drought. During drought, evaporation from open bodies of water increases, while transpiration, particularly from shallow-rooted plants, generally decreases as the near-surface soil moisture is depleted.
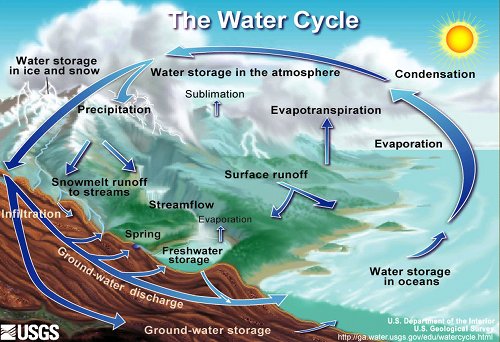
Knowing the amount of precipitation gain and evapotranspiration loss allows agriculturalists to determine the water needs of a crop so that the crop may reach its full production potential in its given environment.
Another important role played by evaporation is that of evaporative cooling, which results from the loss of heat from the evaporating surface due to the loss of the latent heat of evaporation. From a weather perspective, it can alter the temperature characteristics of the surface, thus influencing the heat balance of the surface. This can be particularly important over heavily transpiring plant stands making them cooler than the surroundings. The reduction in evapotranspiration in an urban landscape is considered as one factor causing the urban heat island.
Most mammals use evaporative cooling, either through perspiration or through evaporation of water from their surface, to keep their body temperature from rising to critical levels. (It is why we feel cold when emerging from a shower or swimming pool.) The amount of heat lost through perspiration depends on the evaporation rate, which in turn depends on the air’s humidity and temperature. On hot, humid days, perspiration cannot evaporate rapidly and will remain on our skins, and as a result, we remain hot and feel clammy. Other animal and some plant species also use evaporative cooling to regulate their core temperatures. Evaporative cooling is especially effective on days when the air is hot and humidity is low.
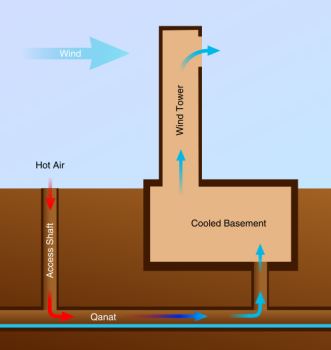
Evaporative cooling has been a common method of cooling buildings for many centuries. Perhaps the earliest application was the windcatcher, invented in Persia (Iran) in ancient times, which takes the form of wind shafts on the roof that catch the wind and pass it over water before channeling the now-cooled air into the building. Other cooling apparatus have also used evaporative cooling for buildings since it is relatively inexpensive and uses much less energy than other mechanical cooling devices. Its major drawbacks are that an abundant water supply is required, which restricts its use in arid regions, and is only efficient when the relative humidity is low, making it less attractive in humid climates such as the US South.
 |
 |
The windcatcher of Dowlat Abad in Yazd, |
Diagram of a building cooled by a qanat and wind tower |
On a hot July day, we surely welcome the role of evaporative cooling as we splash in lakes and pools. Even pouring water over our heads can give cool comfort on a hot afternoon. Even those of us who would normally abhor getting caught in a lawn sprinkler, relish the thought during the hot summer.
 |
To Purchase Notecard, |
Now Available! Order Today! | |
 |
 |
Now |
The BC Weather Book: |


